


Adjudication Guideline
Read MoreThis comprehensive US abdomen guideline outlines the adjudication process for related services, detailing coverage requirements, medical necessity criteria, and non-covered services across various categories. This document applies to all providers operating under agreements with NAS Administration Services Company LLC and Neuron LLC (collectively, NAS Neuron). Please note that the content is subject to periodic review and revision.
Ultrasound imaging is a noninvasive medical test that helps physicians diagnose and treat medical conditions. It is safe and painless. It produces pictures of the inside of the body using sound waves. Ultrasound imaging is also called sonography. It uses a small probe called a transducer and gel placed directly on the skin. High-frequency sound waves travel from the probe through the gel into the body. The probe collects the sounds that bounce back. A computer uses those sound waves to create an image. Ultrasound exams do not use radiation (x-rays). Because ultrasound captures images in real-time, it can show the structure and movement of the body's internal organs. The images can also show blood flowing through blood vessels.
The indications for performing an abdominal ultrasound include, but are not limited to, the following:
1. Evaluation of Pain and Symptoms:
2. Assessment of Palpable or Clinical Findings:
3. Investigation of Abnormal Findings:
4. Monitoring and Follow-up:
5. Trauma Assessment:
6. Oncological Evaluation:
7. Renal and Urinary Tract Assessment:
8. Fluid Detection:
9. Congenital and Pediatric Indications:
10. Transplant Evaluation:
11. Procedural Support:
1. A clear and specific clinical indication (e.g., abdominal pain, palpable mass, jaundice).
2. Relevant history, signs, or symptoms supporting the medical necessity of the ultrasound.
3. Abnormal physical examination findings or prior diagnostic test results (labs or imaging) suggesting abdominal or retroperitoneal pathology
A complete abdominal ultrasound involves a comprehensive evaluation of the major abdominal organs and structures.
The following structures must be evaluated and documented (unless contraindicated or non-visualized, with explanation):
Documentation Requirements:
A limited abdominal ultrasound focuses on specific organs, areas, or symptoms, rather than evaluating the entire abdomen.
Examples of limited study indications include:
Documentation Requirements:
Note - All diagnostic ultrasound examinations require permanently recorded images with measurements, when such measurements are clinically indicated.
Ultrasound examinations are only billable when the clinical indication justifies a thorough evaluation of the relevant organ(s) or anatomical region(s), accompanied by adequate image documentation and a finalized, signed report.
Use of ultrasound, without thorough evaluation of organ(s) or anatomic region, image documentation, and final, written report, is not separately reportable and is considered part of the consultation during which it was performed.
Ultrasound examinations will not be covered in the following situations:
CPT codes covered in the above section: -
| CPT_CODE | FULL_DESCRIPTION |
|---|---|
| 76700 | Ultrasound, abdominal, real time with image documentation; complete |
| 76705 | Ultrasound, abdominal, real time with image documentation; limited (eg, single organ, quadrant, follow-up) |
| Effective Date: | Revision Date: | Version History: |
|---|---|---|
| 22/12/2025 | N/A | V1.0 |
By accessing these NAS Neuron Adjudication Guidelines, you acknowledge and confirm that you have read, understood, and agreed to the terms outlined in this disclaimer. The information contained in these guidelines is intended solely to outline the adjudication procedures followed by NAS Neuron in the context of medical claims processing. These guidelines are not intended to be comprehensive and must not be construed as clinical treatment guidance. They are provided for general informational purposes only and are not intended to replace the clinical judgment of healthcare professionals. NAS Neuron does not engage in the provision of medical care or clinical decision-making and assumes no responsibility for any treatment decisions made by healthcare providers based on these guidelines. All decisions regarding patient care remain the sole responsibility of the treating healthcare provider. These guidelines do not create any legal rights or obligations for or against NAS Neuron. All content is provided “as is,” without any express or implied warranties, including but not limited to implied warranties of merchantability or fitness for a particular purpose.
Under no circumstances shall NAS Neuron be held liable for any direct, indirect, incidental, special, consequential, or punitive damages arising from the use of, access to, or reliance on these guidelines. This includes, without limitation, damages for loss of profits, business interruption, or loss of data, even if NAS Neuron has been advised of the possibility of such damages. These guidelines are subject to and governed by the applicable laws, decrees, circulars, and regulations of Dubai and the United Arab Emirates. They do not override or replace any applicable laws, formal contractual agreements, or regulatory directives issued by UAE governmental or regulatory authorities. This Adjudication Guideline is the intellectual property of NAS Neuron and may not be copied, reproduced, distributed, or displayed in whole or in part without the express written consent of NAS Neuron. These guidelines incorporate Current Procedural Terminology (CPT®), which is a registered trademark of the American Medical Association (AMA), with all associated codes and descriptions owned by the AMA. NAS Neuron reserves the right to amend, update, or withdraw these guidelines at any time without prior notice.
This comprehensive Helicobacter pylori guideline outlines the adjudication process for related services, detailing coverage requirements, medical necessity criteria, and non-covered services across various categories. This document applies to all providers operating under agreements with NAS Administration Services Company LLC and Neuron LLC (collectively, NAS Neuron). Please note that the content is subject to periodic review and revision.
Helicobacter pylori (H. pylori) is a spiral-shaped bacterium that colonizes the mucosal lining of the stomach and the duodenum—the initial segment of the small intestine. It is a common cause of chronic gastritis and peptic ulcer disease, leading to inflammation and ulceration of the gastric or duodenal mucosa. H. pylori infection affects over half of the global population.
In patients with suspected Helicobacter pylori infection, the following laboratory tests are recommended to establish the diagnosis:
Urea Breath Test (UBT): The urea breath test is a non-invasive, FDA-approved method for both initial diagnosis and post-treatment confirmation of Helicobacter pylori infection in adults and children over 3 years of age. It works by detecting labeled carbon dioxide in the breath, produced when H. pylori urease metabolizes ingested ¹³C- or ¹⁴C-labeled urea. Testing must be performed in a fasting state, with breath sample collection occurring approximately one hour after ingestion. The test is not recommended for children under 3 years, as performance data in this age group are insufficient.
Stool Antigen Test for H. pylori: The stool antigen test is a reliable, non-invasive method for confirming active Helicobacter pylori infection. It detects H. pylori antigens in the stool and is suitable for both initial diagnosis and post-treatment confirmation of eradication. Stool antigen testing is the preferred FDA-approved method for diagnosing H. pylori infection in both pediatric and adult patients.
Serologic Testing (IgG Antibody): H. pylori serology is no longer recommended by the American Gastroenterological Association (AGA) and the American College of Gastroenterology (ACG), as it cannot differentiate between active infection and past exposure. Furthermore, elevated antibody levels can persist for months to years’ post-treatment, making it unreliable for confirming eradication.
| Test | Purpose | When to Use | Key Considerations | Eligibility Criteria |
|---|---|---|---|---|
| Urea Breath Test (UBT) | Detects active H. pylori infection | - Initial diagnosis - Post-treatment confirmation |
- Perform while fasting - FDA-approved for adults and children >3 years - Highly accurate for active infection and eradication verification |
- Adults and children over 3 years old - Must be off antibiotics, PPIs, or bismuth for at least 2 weeks prior to testing |
| Stool Antigen Test for H. pylori | Detects H. pylori antigens in stool | - Initial diagnosis - Post-treatment confirmation |
- Non-invasive - FDA-approved for all ages - Reliable for confirming active infection and eradication |
- All age groups (including infants and children) - Must be off antibiotics, PPIs, or bismuth for at least 2 weeks prior to testing |
| Serologic Testing (IgG Antibody) | Detects antibodies against H. pylori | - Indication of past exposure or current infection (not active) - Not recommended for confirming active infection or eradication |
- Elevated antibodies persist long after treatment - Not recommended by AGA/ACG due to false positives - High rate of false positives may lead to unnecessary treatment |
- All age groups - Not recommended for diagnosis of active infection or post-eradication confirmation |
The American Gastroenterological Association no longer recommends serology (antibody) testing for diagnosing infection or evaluating treatment effectiveness as it is unable to distinguish between active infection and previous exposure to H. Pylori. It does not confirm eradication.
Economic studies showed that the use of stool antigen testing was the most cost-effective approach compared to urea breath testing as recommended in the European guideline with a Grade a, Level 1a evidence.
Fecal Antigen Test (FAT) is considered equivalent to the Urea Breath Test (UBT) in terms of sensitivity and specificity for diagnostic purposes; therefore, coverage will be restricted to Fecal Antigen Test only.
Non-specific Dyspepsia: In the absence of alarm symptoms (e.g., anemia, gastrointestinal bleeding, weight loss, or obstruction), H. pylori testing is not necessary for dyspepsia.
Patients already on therapy: Testing is not indicated in patients who have already undergone treatment for H. pylori unless confirming eradication.
Routine Screening: H. pylori testing is not recommended for routine screening of asymptomatic individuals or those without a history of ulcers or related conditions.
Simultaneous urea breath testing and stool antigen testing for H. pylori is not considered medically necessary, as concurrent testing with both methods is not required.
Evaluating infantile colic.
New-onset dyspepsia in persons aged 60 years or older.
Prediction of the risk of gastric cancer or irritable bowel syndrome
Screening of asymptomatic persons for H. pylori infection.
American Gastroenterological Association no longer recommends serology (antibody) testing for diagnosing infection or evaluating treatment effectiveness as it is unable to distinguish between active infection and previous exposure to H. Pylori.
Based on international recommendations, H. pylori serology testing & UBT C14 is not eligible for coverage.
CPT codes covered in the above section: -
| CPT_CODE | FULL_DESCRIPTION |
|---|---|
| 83009 | Helicobacter pylori, blood test analysis for urease activity, non-radioactive isotope (eg, C-13) |
| 83013 | Helicobacter pylori; breath test analysis for urease activity, non-radioactive isotope (eg, C-13) |
| 83014 | Helicobacter pylori; drug administration |
| 86677 | Antibody; Helicobacter pylori |
| 87338 | Infectious agent antigen detection by immunoassay technique, (eg, enzyme immunoassay [EIA], enzyme-linked immunosorbent assay [ELISA], immunochemiluminometric assay [IMCA]) qualitative or semiquantitative, multiple-step method; Helicobacter pylori, stool |
| 87339 | Infectious agent antigen detection by immunoassay technique, (eg, enzyme immunoassay [EIA], enzyme-linked immunosorbent assay [ELISA], immunochemiluminometric assay [IMCA]) qualitative or semiquantitative, multiple-step method; Helicobacter pylori |
| 78267 | Urea breath test, C-14 (isotopic); acquisition for analysis |
| 78268 | Urea breath test, C-14 (isotopic); analysis |
| Effective Date: | Revision Date: | Version History: |
|---|---|---|
| 22/12/2025 | N/A | V1.0 |
By accessing these NAS Neuron Adjudication Guidelines, you acknowledge and confirm that you have read, understood, and agreed to the terms outlined in this disclaimer. The information contained in these guidelines is intended solely to outline the adjudication procedures followed by NAS Neuron in the context of medical claims processing. These guidelines are not intended to be comprehensive and must not be construed as clinical treatment guidance. They are provided for general informational purposes only and are not intended to replace the clinical judgment of healthcare professionals. NAS Neuron does not engage in the provision of medical care or clinical decision-making and assumes no responsibility for any treatment decisions made by healthcare providers based on these guidelines. All decisions regarding patient care remain the sole responsibility of the treating healthcare provider. These guidelines do not create any legal rights or obligations for or against NAS Neuron. All content is provided “as is,” without any express or implied warranties, including but not limited to implied warranties of merchantability or fitness for a particular purpose. In cases where the payer has issued separate or specific clinical guidelines, the payer’s guidelines shall take precedence and will be applied accordingly.
Under no circumstances shall NAS Neuron be held liable for any direct, indirect, incidental, special, consequential, or punitive damages arising from the use of, access to, or reliance on these guidelines. This includes, without limitation, damages for loss of profits, business interruption, or loss of data, even if NAS Neuron has been advised of the possibility of such damages. These guidelines are subject to and governed by the applicable laws, decrees, circulars, and regulations of Dubai and the United Arab Emirates. They do not override or replace any applicable laws, formal contractual agreements, or regulatory directives issued by UAE governmental or regulatory authorities. This Adjudication Guideline is the intellectual property of NAS Neuron and may not be copied, reproduced, distributed, or displayed in whole or in part without the express written consent of NAS Neuron. These guidelines incorporate Current Procedural Terminology (CPT®), which is a registered trademark of the American Medical Association (AMA), with all associated codes and descriptions owned by the AMA. NAS Neuron reserves the right to amend, update, or withdraw these guidelines at any time without prior notice.
This comprehensive vitamin D guideline outlines the adjudication process for vitamin D services, detailing coverage requirements, medical necessity criteria, and non-covered services across various categories. This document applies to all providers operating under agreements with NAS Administration Services Company LLC and Neuron LLC (collectively, NAS Neuron). Please note that the content is subject to periodic review and revision.
Vitamin D deficiency is a condition where the body lacks adequate vitamin D to maintain normal calcium metabolism, bone health, and muscular function. It impairs bone mineralization and increases the risk of skeletal and possibly non skeletal disorders.
Testing for Vitamin D deficiency is considered medically necessary in patients with any of the following conditions:
| Patient Type | Coverage criteria |
|---|---|
| Children (Up to 18 years of age) |
•Deformed bones (bowlegs or knock knees) • Poor growth, delayed fontanelle closure • Delayed walking or a waddling gait • Tender or swollen joints, classically the wrists or costochondral junctions • Bone pain and tenderness • Delayed eruption of teeth or enamel hypoplasia • Carpopedal spasm, seizures or irritability • Breathing difficulties (apnoea or stridor) • All children with suspected metabolic bone disease, with relevant clinical features • Malabsorption syndromes |
| Adult |
• Bone pain • Proximal myopathy • Low bone mineral density +- fracture • Laboratory features such as hypocalcaemia, hypophosphataemia and increased ALP are often a late presenting feature of vitamin D deficiency • Osteoporosis or significant risk of developing osteoporosis (e.g.: malabsorption, CF) |
Repeat blood testing for vitamin D is only required for a small number of clinical indications.
Routine monitoring of vitamin D levels in patients taking supplements is generally unnecessary, except under specific circumstances outlined below.
| Clinical Situation | Recommendation |
|---|---|
Vitamin D therapy and patient in one of the following categories (usually in conjunction with secondary care): • Osteoporosis• Malabsorption (to include cystic fibrosis and coeliac disease) • Chronic hepatic and renal disease • Taking anticonvulsants or similar medications • Children with clinical rickets |
Covered only if repeated after 3-8 months on recommended replacement dose where baseline was low. Annual monitoring for patients on adequate replacement. Repeats will not be allowed before 3 months. All requests for repeat measurement will be reviewed. The clinical indication for repeat testing should be clearly mentioned. |
Vitamin D therapy for whatever clinical indication where baseline Vitamin D concentration was low. |
Do not retest, unless patient’s symptoms have not resolved or otherwise clinically indicated. Repeats will not be allowed before 3 months. All requests for repeat measurement will be reviewed. Clinical indication for repeat testing should be clearly mentioned. |
Risk factors for vitamin D deficiency" refer to conditions, behaviors, or characteristics that increase the likelihood of an individual having low serum levels of vitamin D.
Identifying these risk factors is important for guiding screening, prevention, and treatment efforts.
| Inadequate UV light exposure | Gastrointestinal | Metabolic risk |
|---|---|---|
| • Occlusive garments • Pigmented skin • Institutionalised or housebound |
• Vegetarian (or fish-free diet) • Malabsorption, short bowel or liver disease • Cholestyramine use |
• Older people • Drugs (Rifampicin, anticonvulsants, antiretroviral therapy, high dose glucocorticoids) • Multiple, short interval pregnancies • Prolonged breast feeding without vitamin D supplementation |
CPT codes covered in the above section: -
| CPT_CODE | FULL_DESCRIPTION |
|---|---|
| 82306 | Vitamin D; 25 hydroxy, includes fraction(s), if performed |
| 82652 | Vitamin D; 1, 25 dihydroxy, includes fraction(s), if performed |
| Effective Date: | Revision Date: | Version History: |
|---|---|---|
| 22/12/2025 | N/A | V1.0 |
By accessing these NAS Neuron Adjudication Guidelines, you acknowledge and confirm that you have read, understood, and agreed to the terms outlined in this disclaimer. The information contained in these guidelines is intended solely to outline the adjudication procedures followed by NAS Neuron in the context of medical claims processing. These guidelines are not intended to be comprehensive and must not be construed as clinical treatment guidance. They are provided for general informational purposes only and are not intended to replace the clinical judgment of healthcare professionals. NAS Neuron does not engage in the provision of medical care or clinical decision-making and assumes no responsibility for any treatment decisions made by healthcare providers based on these guidelines. All decisions regarding patient care remain the sole responsibility of the treating healthcare provider. These guidelines do not create any legal rights or obligations for or against NAS Neuron. All content is provided “as is,” without any express or implied warranties, including but not limited to implied warranties of merchantability or fitness for a particular purpose.
Under no circumstances shall NAS Neuron be held liable for any direct, indirect, incidental, special, consequential, or punitive damages arising from the use of, access to, or reliance on these guidelines. This includes, without limitation, damages for loss of profits, business interruption, or loss of data, even if NAS Neuron has been advised of the possibility of such damages. These guidelines are subject to and governed by the applicable laws, decrees, circulars, and regulations of Dubai and the United Arab Emirates. They do not override or replace any applicable laws, formal contractual agreements, or regulatory directives issued by UAE governmental or regulatory authorities. This Adjudication Guideline is the intellectual property of NAS Neuron and may not be copied, reproduced, distributed, or displayed in whole or in part without the express written consent of NAS Neuron. These guidelines incorporate Current Procedural Terminology (CPT®), which is a registered trademark of the American Medical Association (AMA), with all associated codes and descriptions owned by the AMA. NAS Neuron reserves the right to amend, update, or withdraw these guidelines at any time without prior notice.
This comprehensive NCS & EMG testing guideline outlines the adjudication process for NCS & EMG testing services, detailing coverage requirements, medical necessity criteria, and non-covered services across various categories. This document applies to all providers operating under agreements with NAS Administration Services Company LLC and Neuron LLC (collectively, NAS Neuron). Please note that the content is subject to periodic review and revision.
Electrodiagnostic tests are electrophysiological techniques used to evaluate the function and integrity of neuromuscular components, including peripheral nerves, nerve roots, plexuses, the neuromuscular junction (NMJ), and muscles.
These tests are classified into 2 primary types—needle electromyography (EMG) and nerve conduction studies (NCS). Both NCS and EMG record electrical activity in the peripheral nerves and muscles.
A nerve conduction study (NCS) is a medical test used to assess the health and functioning of the peripheral nervous system, which includes the nerves outside the brain and spinal cord. It is a diagnostic procedure that helps in evaluating nerve damage, detecting nerve-related disorders, and determining the extent and location of nerve injuries or abnormalities.
NCS is classified into 3 main types—motor, sensory, and mixed.
To determine the motor conduction velocity of a motor nerve, electrical stimulation is applied at multiple points along the nerve, and the resulting responses are recorded from the corresponding muscle.
To determine the sensory conduction velocity of a nerve, electrical stimulation is applied near the sensory nerve, and the resulting response is recorded at a different site along the nerve.
Mixed nerve conduction studies (MNCS) evaluate both motor and sensory components of a mixed nerve by applying electrical stimulation at a distal site and recording the resulting mixed nerve action potentials (MNAPs) from a more proximal point along the same mixed nerve.
Electromyography (EMG) is a diagnostic procedure that records the electrical activity of muscles at rest and during voluntary contraction. It is used to evaluate the integrity and function of muscles and their innervating nerves. EMG is indicated in the assessment of neuromuscular junction disorders, peripheral nerve pathologies, and primary muscle diseases.
NCV studies are effective in assessing the pathophysiology of peripheral nerve disorders. Electrodiagnostic studies are typically performed as an adjunct to clinical examination. NCS is valuable in confirming clinical diagnoses and can also help identify subclinical conditions, localize focal nerve abnormalities, measure severity, and characterize the nature of the lesion.
Common indications include focal nerve entrapments, demyelinating mononeuropathy, axonal loss mononeuropathy, preganglionic lesions, and both demyelinating and axonal polyneuropathies.
Indications for Nerve Conduction Studies (NCS) in Neurological Conditions
Common indications for NCS include the evaluation of:
Indications for EMG in Suspected Neuromuscular Disorders
EDX (electrodiagnostic) testing is used to evaluate the integrity and function of the peripheral nervous system (most cranial nerves, spinal roots, plexi, and nerves), neuromuscular junction, muscles, and the central nervous system (brain and spinal cord).
Codes 95907-95913 describe one or more nerve conduction studies.
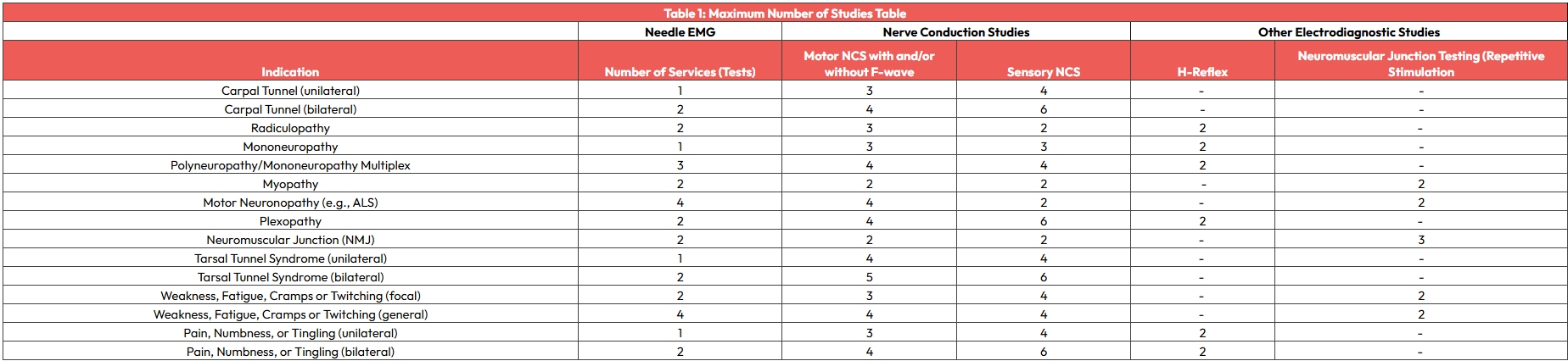
For the purposes of coding, a single conduction study is defined as a sensory conduction test, a motor conduction test with or without an F wave test, or an H-reflex test.
Each type of study (sensory, motor with or without F wave, H-reflex) for each nerve includes all orthodromic and antidromic impulses associated with that nerve and constitutes a distinct study when determining the number of studies in each grouping (eg, 1-2 or 3-4 nerve conduction studies).
Each type of nerve conduction study is counted only once when multiple sites on the same nerve are stimulated or recorded.
The following are considered not medically reasonable and necessary:
CPT codes covered in the above section: -
| CPT_CODE | FULL_DESCRIPTION |
|---|---|
| 95907 | Nerve conduction studies; 1-2 studies |
| 95908 | Nerve conduction studies; 3-4 studies |
| 95909 | Nerve conduction studies; 5-6 studies |
| 95910 | Nerve conduction studies; 7-8 studies |
| 95911 | Nerve conduction studies; 9-10 studies |
| 95912 | Nerve conduction studies; 11-12 studies |
| 95913 | Nerve conduction studies; 13 or more studies |
| 95860 | Needle electromyography; 1 extremity with or without related paraspinal areas |
| 95861 | Needle electromyography; 2 extremities with or without related paraspinal areas |
| 95863 | Needle electromyography; 3 extremities with or without related paraspinal areas |
| 95864 | Needle electromyography; 4 extremities with or without related paraspinal areas |
| 95885 | Needle electromyography, each extremity, with related paraspinal areas, when performed, done with nerve conduction, amplitude and latency/velocity study; limited (List separately in addition to code for primary procedure) |
| 95886 | Needle electromyography, each extremity, with related paraspinal areas, when performed, done with nerve conduction, amplitude and latency/velocity study; complete, five or more muscles studied, innervated by three or more nerves or four or more spinal levels (List separately in addition to code for primary procedure) |
| Effective Date: | Revision Date: | Version History: |
|---|---|---|
| 22/12/2025 | N/A | V1.0 |
By accessing these NAS Neuron Adjudication Guidelines, you acknowledge and confirm that you have read, understood, and agreed to the terms outlined in this disclaimer. The information contained in these guidelines is intended solely to outline the adjudication procedures followed by NAS Neuron in the context of medical claims processing. These guidelines are not intended to be comprehensive and must not be construed as clinical treatment guidance. They are provided for general informational purposes only and are not intended to replace the clinical judgment of healthcare professionals. NAS Neuron does not engage in the provision of medical care or clinical decision-making and assumes no responsibility for any treatment decisions made by healthcare providers based on these guidelines. All decisions regarding patient care remain the sole responsibility of the treating healthcare provider. These guidelines do not create any legal rights or obligations for or against NAS Neuron. All content is provided “as is,” without any express or implied warranties, including but not limited to implied warranties of merchantability or fitness for a particular purpose.
UnderUnder no circumstances shall NAS Neuron be held liable for any direct, indirect, incidental, special, consequential, or punitive damages arising from the use of, access to, or reliance on these guidelines. This includes, without limitation, damages for loss of profits, business interruption, or loss of data, even if NAS Neuron has been advised of the possibility of such damages. These guidelines are subject to and governed by the applicable laws, decrees, circulars, and regulations of Dubai and the United Arab Emirates. They do not override or replace any applicable laws, formal contractual agreements, or regulatory directives issued by UAE governmental or regulatory authorities. This Adjudication Guideline is the intellectual property of NAS Neuron and may not be copied, reproduced, distributed, or displayed in whole or in part without the express written consent of NAS Neuron. These guidelines incorporate Current Procedural Terminology (CPT®), which is a registered trademark of the American Medical Association (AMA), with all associated codes and descriptions owned by the AMA. NAS Neuron reserves the right to amend, update, or withdraw these guidelines at any time without prior notice.
This comprehensive billing guideline for room & board with infusion & injection services outlines the adjudication process for related services, detailing coverage requirements, medical necessity criteria, and non-covered services across various categories. This document applies to all providers operating under agreements with NAS Administration Services Company LLC and Neuron LLC (collectively, NAS Neuron). Please note that the content is subject to periodic review and revision.
This adjudication rule outlines the reporting requirements for therapeutic, prophylactic, and diagnostic injections and infusions (excluding chemotherapy) within the Outpatient–Short Stay (OP–Short Stay) category. It is designed to standardize the processing of infusion and injectable drug cases and ensure compliance with established drug labeling guidelines.
Injections codes are differentiated based on the route of administration, including Intravenous (IV), subcutaneous (SC), intramuscular (IM), and intra-arterial injections.
Following services are included in infusion or injection or hydration and should not be reported separately:
| S.no | Drug / Service | CPT codes |
|---|---|---|
| 1 | Hydration (Normal Saline/ Ringer lactate, etc.) | 96360-96361 |
| 2 | Therapeutic/ Diagnostic drugs (Antibiotics, Steroids, NSAIDS, Narcotic analgesics, Antiemetics, etc.) | 96365-96379 |
Requests for the example codes listed below must be submitted under outpatient only. DSL 17.24 and DSL 15, Per diem 24 should not be reported, as board or per diem charges are not payable for these services,
| S.no | Drug / Service | CPT codes |
|---|---|---|
| 1 | Arthrocentesis, Aspiration/ Injection in joints (small, intermediate) | 20600-20606 |
| 2 | Intravitreal injections (Anti VEGF agents) | 67028 |
| DSL Code | Description |
|---|---|
| 15 | Per diem -Observation <6 hours |
| 24 | Per diem – Short stay |
| 17.24 | Short Stay - Hourly Rate |
The following services are included under the above DSL codes when coded or billed with the relevant CPT codes,
| Bed / Room | Bed/room use of a hospital bed or chair (not inpatient room & board) |
| Documentation & admin costs | Documentation & admin costs - Charting, patient intake, discharge planning |
| Use of hospital facilities | Use of hospital facilities Utilities, maintenance, support staff |
| Other services | Nursing services Routine monitoring, vitals, IV setup, patient assessments |
| Basic medical supplies/consumables | Basic medical supplies Standard consumables: gloves, syringes, tubing, saline flushes, etc. |
Please ensure that the following details are submitted, wherever applicable, when submitting a request with the above DSL code to facilitate accurate adjudication.
| Drug administration | Pertinent CPT code |
| Drugs/biologics | Drug code (DDC/ Riayati) |
| Labs and imaging | CPT codes for labs, X-rays, CTs, etc. |
| Physician services | Billed separately (If applicable) |
| Special equipment or monitoring | If used, may be billed separately |
Patient treatment supplies (including for example: elastic stockings, ace bandages, gauze, syringes, diabetic test strips, and like products; non-prescription drugs and treatments,) excluding supplies required as a result of Healthcare Services rendered during a Medical Emergency.
Any inpatient treatment, investigations or other procedures, which can be carried out on outpatient basis without jeopardizing the Insured Person’s health.
| Effective Date: | Revision Date: | Version History: |
|---|---|---|
| 19/01/2026 | N/A | V1.0 |
By accessing these NAS Neuron Adjudication Guidelines, you acknowledge and confirm that you have read, understood, and agreed to the terms outlined in this disclaimer. The information contained in these guidelines is intended solely to outline the adjudication procedures followed by NAS Neuron in the context of medical claims processing. These guidelines are not intended to be comprehensive and must not be construed as clinical treatment guidance. They are provided for general informational purposes only and are not intended to replace the clinical judgment of healthcare professionals. NAS Neuron does not engage in the provision of medical care or clinical decision-making and assumes no responsibility for any treatment decisions made by healthcare providers based on these guidelines. All decisions regarding patient care remain the sole responsibility of the treating healthcare provider. These guidelines do not create any legal rights or obligations for or against NAS Neuron. All content is provided “as is,” without any express or implied warranties, including but not limited to implied warranties of merchantability or fitness for a particular purpose.
Under no circumstances shall NAS Neuron be held liable for any direct, indirect, incidental, special, consequential, or punitive damages arising from the use of, access to, or reliance on these guidelines. This includes, without limitation, damages for loss of profits, business interruption, or loss of data, even if NAS Neuron has been advised of the possibility of such damages. These guidelines are subject to and governed by the applicable laws, decrees, circulars, and regulations of Dubai and the United Arab Emirates. They do not override or replace any applicable laws, formal contractual agreements, or regulatory directives issued by UAE governmental or regulatory authorities. This Adjudication Guideline is the intellectual property of NAS Neuron and may not be copied, reproduced, distributed, or displayed in whole or in part without the express written consent of NAS Neuron. These guidelines incorporate Current Procedural Terminology (CPT®), which is a registered trademark of the American Medical Association (AMA), with all associated codes and descriptions owned by the AMA. NAS Neuron reserves the right to amend, update, or withdraw these guidelines at any time without prior notice.
This guideline outlines the adjudication and utilization principles for Transthoracic Echocardiography (TTE), Doppler Echocardiography, Transesophageal Echocardiography (TEE), and Stress Echocardiography for health plans administered by NAS and Neuron. The guideline is aligned with UAE regulatory expectations (DOH/DHA), international cardiology standards (ASE, ESC, ACC/AHA), and fair medical necessity determination principles. Fetal echocardiography procedures are excluded from the scope of this guideline.
Echocardiography is a non-invasive diagnostic ultrasound examination of the heart and great vessels using high-frequency sound waves to generate real-time images of cardiac structures and assess blood flow using Doppler techniques. An echocardiographic examination may include:
The extent of imaging performed shall be based on the clinical indication, patient condition, and technical feasibility, in accordance with recognized clinical practice guidelines.
TTE is a non-invasive ultrasound examination performed via the chest wall and is the primary imaging modality for assessment of:
TTE is considered a first-line diagnostic tool for many cardiac conditions when clinically indicated.
TEE utilizes an esophageal transducer to obtain high-resolution images and is indicated when TTE images are non-diagnostic or when detailed evaluation of specific cardiac structure is required. Conscious sedation and continuous monitoring are required. Contraindications include active upper gastrointestinal bleeding and significant esophageal pathology.
Stress echocardiography evaluates myocardial function under stress conditions induced by exercise or pharmacologic agents. It is an accepted non-invasive modality for ischemia assessment, myocardial viability, valvular assessment, and prognostic evaluation in appropriately selected patients.
Echocardiography is considered medically necessary when supported by:
Echocardiography may be ordered as a first-line investigation when clinically appropriate. Coding must reflect the services actually performed and documented, to the highest level of specificity.
Echocardiography may not be eligible for coverage in the following circumstances:
A complete transthoracic echocardiography study includes:
In addition to the above, a complete study generally includes assessment of the following cardiac structures such as.
If certain structures cannot be adequately visualized due to patient-specific or technical limitations, this must be clearly documented in the report and does not automatically preclude billing of a complete study, provided a comprehensive imaging attempt was made. Congenital heart disease, when identified or suspected, shall be reported using the appropriate congenital echocardiography CPT codes (93303–93304), subject to policy benefits. For further details on documentation, please refer to the requirements as outlined in the documentation requirements section below.
A limited echocardiography study is appropriate when:
The scope of a limited study must be clearly documented and aligned with the clinical indication.
Doppler and Add-On Services
CPT 93306 includes spectral and color Doppler imaging. Add-on Doppler codes (93320, 93321, 93325) shall only be reported when Doppler services are performed independently of bundled services, in accordance with CPT coding rules. Unbundling or denial of bundled Doppler services is not permitted when appropriately documented within CPT 93306.
Echocardiography reports shall include, where clinically applicable, but not limited to:
Repeat echocardiography is considered medically appropriate when supported by:
| CPT Code | Description |
|---|---|
| 93306 | Echocardiography, transthoracic (TTE), complete with 2D, M-mode, spectral Doppler, and color Doppler<6 hours |
| 93307 | Echocardiography, transthoracic, complete with 2D and M-mode, without spectral or color Doppler |
| 93308 | Echocardiography, transthoracic, follow-up or limited study |
| ✚ 93320 | Doppler echocardiography, pulsed/continuous wave with spectral display; complete (add-on) |
| ✚ 93321 | Doppler echocardiography, follow-up or limited study (add-on) |
| ✚ 93325 | Doppler color flow velocity mapping (add-on) |
| 93350 | Transthoracic echocardiography during rest and stress test, with interpretation and report |
Coverage determinations shall adhere to:
Providers retain the right to clinical clarification, peer-to-peer discussion, and appeal in cases of disagreement regarding medical necessity or coding interpretation.
| Effective Date: | Revision Date: | Version History: |
|---|---|---|
| 09/02/2026 | N/A | V1.0 |
By accessing these NAS Neuron Adjudication Guidelines, you acknowledge and confirm that you have read, understood, and agreed to the terms outlined in this disclaimer. The information contained in these guidelines is intended solely to outline the adjudication procedures followed by NAS Neuron in the context of medical claims processing. These guidelines are not intended to be comprehensive and must not be construed as clinical treatment guidance. They are provided for general informational purposes only and are not intended to replace the clinical judgment of healthcare professionals. NAS Neuron does not engage in the provision of medical care or clinical decision-making and assumes no responsibility for any treatment decisions made by healthcare providers based on these guidelines. All decisions regarding patient care remain the sole responsibility of the treating healthcare provider. These guidelines do not create any legal rights or obligations for or against NAS Neuron. All content is provided “as is,” without any express or implied warranties, including but not limited to implied warranties of merchantability or fitness for a particular purpose.
Under no circumstances shall NAS Neuron be held liable for any direct, indirect, incidental, special, consequential, or punitive damages arising from the use of, access to, or reliance on these guidelines. This includes, without limitation, damages for loss of profits, business interruption, or loss of data, even if NAS Neuron has been advised of the possibility of such damages. These guidelines are subject to and governed by the applicable laws, decrees, circulars, and regulations of Dubai and the United Arab Emirates. They do not override or replace any applicable laws, formal contractual agreements, or regulatory directives issued by UAE governmental or regulatory authorities. This Adjudication Guideline is the intellectual property of NAS Neuron and may not be copied, reproduced, distributed, or displayed in whole or in part without the express written consent of NAS Neuron. These guidelines incorporate Current Procedural Terminology (CPT®), which is a registered trademark of the American Medical Association (AMA), with all associated codes and descriptions owned by the AMA. NAS Neuron reserves the right to amend, update, or withdraw these guidelines at any time without prior notice.
